The Theory
- There exists a family of naturally occurring halogenated acetates that are known to enhance the function of mitochondria.
- When these compounds are present in cancerous cells they have the potential to reactivate certain functions of the mitochondria, triggering apoptosis.
- For the process to be effective, oxygen must be present in the cell. For hypoxic tumors, this necessitates the introduction of high pressure oxygen therapy to saturate the cells with oxygen.
- A natural version of this process is already in effect for marine life.
The current cancer paradigm
There are over 200 types of human cancer. This is because there are over 200 different human cell types that can become cancerous. Depending on where they occur in the body, they produce different symptoms. A cancerous tumor could be composed of the same cell type, but will require different treatment if it occurs in the brain, lungs or liver.
This has lead to much research in chemotherapy compounds that target specific cell types and tumor locations. Individuals can also react differently to the same chemo drugs, so detailed genetic profiling must be done to determine if the drugs will be effective at all. Even with all the money spent researching these exotic compounds and investigating the genetic profile of the patient and tumor, the success rate of these drugs are low enough that oncologists will refuse the same treatment they’re prescribing.
Even if the chemo is successful in eradicating the tumor, most of the drugs used in the treatment have seriously debilitating side effects and are also known carcinogens that can trigger the further development of cancer later on in life.
At this point in time, there are few accepted avenues for treatment that don’t involve carcinogenic chemotherapy, damaging radiation or invasive surgery.
The common ground
Cancer is considered to be a modern day plague, brought on our diets full of artificial compounds, our high stress lifestyles and our increased exposure to environmental toxins. While the majority of the current research involves studying the genetic causes of cancer and how those genes interact with chemotherapy drugs, recent research has identified alternative routes for attacking cancerous cells that don’t rely on toxic and carcinogenic compounds, radiation or surgery. Instead, these compounds activate natural pathways of the mitochondria that can trigger the programmed cell death of cancerous cells, also known as apoptosis.
In apoptosis the cell doesn’t simply die, but rather it dissolves into distinct cellular fragments which are then processed by other cells in the body safely. In the case of cell that had been disrupted by a toxic or carcinogenic chemical, the process of apoptosis allows the compound to remain safely enclosed in cellular fragments of the former cell when engulfed by the body’s cellular recycling system. This differs from when a cell dies due to injury or necrosis. In that case the damaged cellular membrane simply releases its contents into the extracellular fluid, potentially damaging nearby healthy cells.
From this it can be seen that the function of the mitochondria is not only to provide energy for the cell and play a roll in programmed cell death, but they also play an important roll in supporting the health of differentiated tissue by ensuring cell death occurs in an orderly manner that doesn’t affect nearby tissue.
Being able to trigger this cellular pathway is a universal means of treating cancer, one that doesn’t rely on detailed genetic profiling to target the use of toxic and damaging chemicals on specific types of cancer, radiation or surgery, but instead uses a natural cellular process to deal with cancer in a non-invasive manner.
The Discovery
In January of 2007, the University of Alberta reported that a drug (DCA or the salts of the dicholoroacetate ion) previously used in humans to treat a rare metabolic disorder was also biologically active in the treatment of various forms of cancer.
Their theory of how the drug operates involves the Warburg Effect, a known phenomenon in cancerous cells where the cells are hypoxic and do not use oxygen and the mitochondria for energy generation, instead using other pathways that harvest energy from nearby healthy cells. According to their research, DCA appears to reactivate mitochondrial function, which also reactivates the mitochondria’s ability to regulate cellular functions, including apoptosis. This results in cell death for cancerous cells, leaving healthy cells undamaged.
I’m going to expand on their theory by examining certain properties of DCA which could be responsible for its biological activity in the treatment of cancers as well as providing examples of its existing activity in marine environments. The goal being to expand this theory into a laboratory environment where it can be rigorously tested.
Current Status of Clinical Trials in Canada
On May 12th of 2010, the Department of Medicine released the results of their Phase II clinical trials completed in conjunction with Health Canada, involving in vivo (within the living) testing of DCA. The results were found to be in agreement with earlier in vitro (within glass) studies. They have since begun seeking funding for the Phase III trials. These drug trials are prohibitively expensive and are normally funding by companies within the pharmaceutical industry interested in capitalizing on the development of new drugs. Since DCA is a cheap and off-patent compound, a successful Phase III trial could spell the end for a number of lucrative drug lines currently used to aggressively treat cancer. Due to this economic factor, the funding for the Phase III trials is largely being compiled with donations from private individuals.
The use and availability of DCA is regulated for a reason. People attempting to take DCA without proper knowledge of dosage or even the proper form of the compound to be taken run the risk of doing major damage to their health. Side effects from exceeding a safe daily dosage can damage and interfere with nerve cells. At high enough doses, DCA itself has been shown to display cancer-causing properties.
There is also the danger of those unfamiliar with chemistry to seek out the acid form of DCA instead of a salt form such as sodium or potassium dichloroacetate.
I do not advocate the personal use of pure DCA salts by any readers without the oversight of a well-informed medical professional capable of dealing with and addressing any possible complication that may arise.
I’m currently aware of only one organization currently using DCA to treat cancer. The Medicor Cancer Center in Toronto has been using DCA since 2007, treating patients with several different types of cancer. While having published only one paper pertaining to their results, they have made the results of their clinical use available online and found them to be in agreement with the University of Alberta’s studies.
Hypothesis on the function of DCA within cancer cells
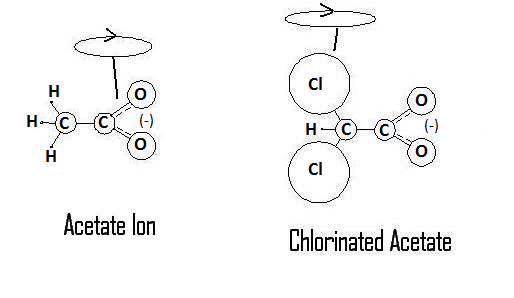
This hypothesis is based around the electrokinetic properties of the dichloroacetate ion. In the following diagram, I have provided an approximated atomic mass ratio comparison of the acetate and dichloroacetate ions, as well outlined the possible spin states that this arrangement of atoms could produce.
The ratios of masses are not intended to be shown to scale, but are there to simply illustrate the differences. For reference the standard atomic weights for each atom shown are as follows: Hydrogen ~ 1, Carbon ~ 12, Oxygen ~ 16, Chlorine ~ 35. To think of the molecule in terms of its center of mass, the standard acetate ion has a mass ratio of 15:44, split around the carbon-carbon bond. The chlorinated acetate, DCA, has a mass ratio of 76:44 split around the same carbon-carbon bond. As indicated in the diagram by the spin loop, the center of mass for rotation has moved from being centered towards the carboxyl group to the halogenated carbon. This new axis of rotation would give carboxyl group a more reactive presence as it moves in solution, a functional character not present in the normal acetate ion. The change can be demonstrated by something as simple as the odour and taste of each compound. When hydrogenated carbon is the reactive portion of the compound as in the acetate ion, the compound has a potent odour and taste. In DCA however, with the reactive portion of the compound being the carboxyl group, the compound is essentially odourless and tasteless.
DCA Analogues
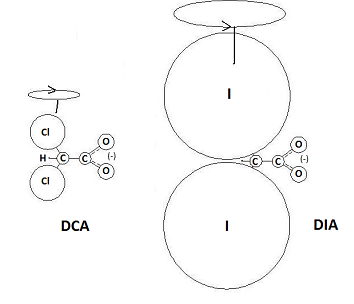
Based on the theory that the source of DCA’s biological activity rests on the functional character of the carboxyl group, it becomes possible to predict structural and functional analogues that should produce a similar if not more potent effect on cancerous cells. By replacing the chlorine with heavier halogens, the effect of the carboxyl group would be magnified and the potency of its interactions within the cell that reactivate mitochondrial function should increase. The most potent of these structural and functional analogues would be the DIA ion or diiodoacetate.
As shown, the atomic mass ratio of the halogenated carbon to the carboxyl group is much larger, which should magnify the electrokinetic influence of the carboxyl group even further. Instead of the 74:44 mass ratio split around DCA’s carbon-carbon bond, there is a 267:44 split around the carbon bond of DIA. To put it in simpler terms, the iodine-based analogue of DCA is capable of producing more torque both on the carboxyl group and by extension, any compound the reactive carboxyl group is interacting with.
Food Sources for DCA/DIA
Acetate (the active ingredient in common vinegar), DCA and DIA are very simple carbon-based compounds. Each bears the same structure, but has slightly different functional characteristics. Acetate is considered to be a biotic or organic compound, as it has been shown to be produced by a natural process, namely, oxidation of alcohol by bacteria.
DCA is currently classified as a xenobiotic compound as it is not thought to occur naturally. One of the only process known to produce DCA as a trace product is the chlorination of water, an artificial process. DCA’s status as a xeno- or abiotic chemical means its use and availability can be heavily regulated, even to the point of denying its use to those who may benefit from established treatment regimes.
DCA, as well as its iodine and bromine-based analogues, can be obtained from a purely natural source as its presence has already been demonstrated in the edible seaweed Asparagopsis taxiformis (R. Moore, Phytochemistry Vol 18 pp 617-620 1979), also known as limu koku, which is a regular part of Hawaiian cuisine.
While currently having only been isolated in one species of seaweed, I believe the presence of these compounds in a species such as Aparagopsis taxiformis should found to be the rule rather than the exception. Seaweeds are natural concentrators of heavy halogens such as iodine, bromine and chlorine from sea water. For these heavy halogens to be used by cellular processes in the creation of potent antioxidants seems a natural use of elemental resources not readily available to terrestrial and freshwater plant life.
DCA is currently a regulated substance and I do not condone the use of DCA without the care of informed medical professionals, edible seaweeds such as Asparagopsis taxiformis that contain a naturally available version DCA and DIA should be safe to consume for those seeking its benefits. Until more research can be done into how processing techniques may alter the compounds present in processed seaweeds, the best option would be to ensure the seaweed is as fresh as possible.
DCA shown to fail with hypoxic tumors
In September of 2010, the University of Guelph announced their findings in regards to DCA and hypoxic tumors. They found that while tumours under normal oxygen conditions responded to DCA as predicted, tumours with restricted access to the blood carried oxygen supply reacted in a completed opposite manner when treated with DCA. Instead of triggering apoptosis, DCA seemed to act in a cytoprotective (cell-protecting) manner. Cancerous cells weren’t dying with the treatment, instead they were getting stronger and showed a decreased response to traditional forms of chemotherapy.
These results seemed to throw a major stumbling block in front of DCA research. Hypoxic tumors are common in cancer pathology as tumors outgrow their blood supply, so treatment with DCA could prove dangerous to a person suffering from a hypoxic tumor. Instead of killing the cancerous cells, DCA treatment could strengthen them, making them more resistant and harder to kill.
While this may have seemed like a roadblock, due to my search for DCA and analogues in seaweed, it gave the last piece I needed for my theory.
A pre-existing niche for dealing with Cancer
Since these compounds seem to appear at the base of the marine food chain, they should be available to all higher marine life. Any and all life that lives in the oceans should be receiving a cocktail of DCA and it’s analogues. But how to address the hypoxic tumor issue?
The answer has to due with pressure.
While we live our lives near or above sea level, our cells receive oxygen through 2 methods. The oxygen is either carried by the hemoglobin in our red blood cells, or it dissolves directly into our blood plasma. The oxygen-carrying capacity of our red blood cells far outweighs the amount of oxygen capable of dissolving in our blood plasma. However, this only remains true at standard atmospheric pressures. As you move below sea level, the concentration of dissolved oxygen increases with the pressure. For creatures like fish that breath underwater, this means that their blood has to do less work, as more of the oxygen dissolves directly into blood plasma.
The effect also applies to temperature. As temperature decreases, so does the water’s ability to retain dissolved oxygen. The effect is so great, there is a species of fish in Antarctica that has no red blood cells to carry oxygen. Known as the crocodile icefish, they are the only known vertebrate that has no red blood cells, the only reason they’re capable of this feat is due to the particular niche they inhabit. At the temperatures provided by the Antarctic, and the pressures provided by the depths live in, the concentration of dissolved oxygen is great they evolved away the need for hemoglobin-based blood cells altogether. They offset this loss of oxygen-carrying cells by having a larger and more powerful heart, with wider capillaries to deal with a greater plasma volume. But for the rest of us, lacking the anti-freeze proteins and gills required to experience the crocodile ice fish’s level of dissolved oxygen, we have to make due with the use of pressure to obtain the dissolve oxygen levels we require. Marine mammals accomplish this by diving.
Baleen whales are known for their immense size, they are the largest creatures on the planet. They’re also known to live as long or longer than humans. This presents modern cancer theory with a bit of a conundrum. Larger animals have potentially more carcinogenic cells, as well as requiring more cell division to grow and sustain their prodigious size. This has lead to a variety of theories regarding the relatively reduced incidence of cancer amongst whales. Not to say that they don’t get cancer at all, it’s just that if their cancer rates matched human rates there wouldn’t be any blue whales.
Instead, it appears that through a combination of diet and environment, blue whales simply evolved into a cancer resistant niche. They consume vast amounts of krill, with a full grown blue whale consuming almost 4 tons of krill in a single day. Krill, in turn, feed on the phytoplankton that should be producing both DCA, DIA and bromine versions as they concentrate these heavy halogens out of seawater. With this kind of daily intake, their cells should be saturated with these compounds.
Blue whales typically feed at depths of 100 meters. At these depths, the pressure is about 11 atmospheres, or 11 times the pressure experienced at sea level. At these depths, the concentration of dissolved oxygen carried by the whales blood plasma instead of their cells is greatly increased. This allows oxygen to penetrate tissues inaccessible to the blood through its networks of capillaries by dissolving directly into the tissues. Through this mixture of a diet supplemented with a known cancer-fighting agent, combined with the increased pressure available in the depths of the oceans, whales seem to have evolved into a cancer resistant niche.
Questions that need to be answered
To replicate the diet and environment accessible to blue whales for human cancer treatment, a combination of dietary supplementation and hyperbaric oxygen treatment should provide the same conditions. But before this theory can be applied in any manner, it must first be tested rigorously. A number of questions must be answered before anything can be done with human trials. However, as an individual, I don’t have the resources to complete this testing on my own. I need assistance from others to answer the following questions:
What is the efficacy of DIA compared to DCA?
Will hypoxic cancer cells respond to a combination of DCA or DIA and hyperbaric oxygen treatment?
What are the threshold levels of both DCA/DIA supplementation and hyperbaric oxygen required for effective treatment?
Are there different threshold levels for different cancers and different stages of cancer progression?
Alternative Options
While the clinical use of DCA/DIA and hyperbaric oxygen requires a number of questions to be answered, it’s possible that these answers may lead to a much simpler way of treating cancers in the long term. Instead of consuming DCA/DIA as a separate supplement, it could be added to the diet by increasing a person’s intake of edible seaweeds. Hyperbaric oxygen treatment could be replicated by extended scuba dives.
Wouldn’t the world be a better place if a cancer diagnosis wasn’t a potential death sentence, but was instead an invitation to spend a few weeks on vacation somewhere tropical, scuba diving and eating seaweeds?